Abstract
Introduction. The optimal treatment of patients with diffuse large B cell (DLBCL) and high-grade B cell (HGBCL) lymphomas with synchronous central nervous system (CNS) and systemic involvement at diagnosis is not well defined. High-dose methotrexate (MTX) administered concurrently with R-CHOP (RM-CHOP) is a commonly used regimen, but data on outcomes achieved with this regimen are limited.
Methods. We included consecutive patients with DLBCL and HGBCL with synchronous CNS and systemic involvement at diagnosis treated with RM-CHOP from 1/2012 - 1/2021. Progression-free survival (PFS) was calculated from the time of diagnosis to either progression or death, and overall survival (OS) from the time of diagnosis to death due to all causes; patients without events were censored at the time of the last follow-up. PFS and OS were estimated through the Kaplan-Meier method.
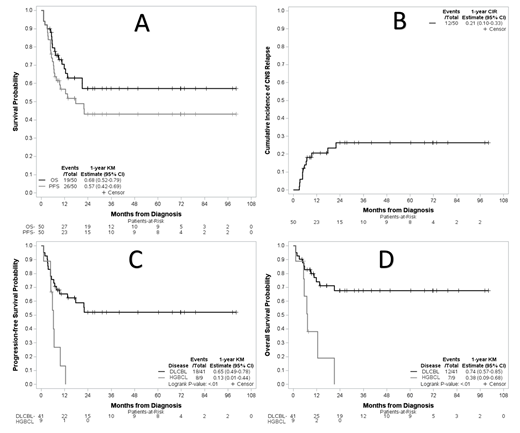
Results. Fifty patients were included. Median age was 62 years (range, 19 - 80), 58% were male, 82% had DLBCL (n=41) and 18% had HGBCL (n=9). Six patients (14%) had MYC with BCL2 and/or BCL6 rearrangements by fluorescence in situ hybridization (available for n=43), and 11 patients (55%) had MYC and BCL2 double-expression by immunohistochemistry (available for n=20). ECOG performance status was ≥2 in 16 patients (36%). LDH was elevated in 41 patients (87%).The IPI score classified 40% and 56% of patients as having intermediate- or high-risk disease, respectively. The median number of extranodal sites was 2 (range, 1-7) with 48% having ≥3 extranodal sites. CNS involvement was parenchymal in 14 patients (28%), leptomeningeal in 28 (56%), or both in 8 (16%). The most common extranodal sites outside the CNS were renal/adrenal (n=6, 12%), paraspinal (n=5, 10%), and testicular (n=4, 8%). The majority of R-CHOP cycles (89%, n=222) included MTX with a median number of MTX-containing R-CHOP cycles administered per patient of 5 (range, 1-6). MTX starting dose was 3.5 g/m 2 in all but 3 patients (range, 1.5 - 2 g/m 2). Intrathecal MTX was administered in 21 patients (42%). Treatment with RM-CHOP was followed by consolidative stem cell transplantation in 15 patients (30%) including 14 autologous (28%) and 1 (2%) allogeneic. The objective response rate following RM-CHOP was 66% including complete response in 60%. With a median follow-up of 31 months (range, 4 - 100), the median PFS and OS were 17.7 months (95% confidence interval (CI), 6.9 - not reached [NR]) and NR (95% CI, 12.5 - NR), respectively, with 1-year PFS and OS of 57% (95% CI, 42-69%) and 68% (95% CI, 52-79%), respectively (Figure A). Twelve patients had CNS relapse/progression with a 1-year cumulative incidence of CNS progression/relapse of 21% (95% CI, 10-33%) (Figure B). Outcomes were particularly poor in patients with HGBCL, with median PFS and OS of 6.3 (95%CI, 1.2 - 9.8) months and 7.3 (95%CI, 1.2 - 21.2) months, and 1-year PFS and OS of 13% (95%CI, 1-44%) and 38% (95% CI, 9-68%), respectively (Figures C and D). Of the 7 patients with HGBCL who relapsed/progressed, 3 had CNS involvement only (1 parenchymal, 2 leptomeningeal), 3 had systemic involvement only, and 1 had both (parenchymal). Three patients received subsequent systemic treatment and only 1 responded (partial response). In patients with DLBCL, with a median follow-up of 33.5 months (range, 4 - 100), the median PFS and OS were both NR (95%CI, 9.5 months - NR and 21 months - NR, respectively) and the 1-year PFS and OS were 65% (95% CI, 49-78%) and 74% (95%CI, 57-85%), respectively. Of the 14 patients with DLBCL who relapsed/progressed, 8 had CNS involvement only (4 parenchymal, 2 leptomeningeal, 2 both) and 6 had systemic involvement only. Nine patients received subsequent systemic treatment of whom 5 patients responded (3 complete and 2 partial responses)
Conclusions. To our knowledge, this is one of the largest studies of patients with DLBCL and HGBCL with synchronous CNS and systemic involvement at diagnosis treated with RM-CHOP. Patients with HGBCL had poor outcomes with median PFS and OS of 6 and 7 months, respectively. Patients with DLBCL had more favorable outcomes with median PFS and OS not reached after a median follow-up of 33.5 months. CNS involvement was more common than isolated systemic involvement at relapse/progression. CNS involvement in aggressive B-cell non-Hodgkin lymphoma at diagnosis remains a major therapeutic challenge, dictates clinical outcomes, and requires more effective treatment options.
Dotson: AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Bond: Kite/Gilead: Honoraria. Epperla: Genzyme: Honoraria; Karyopharm: Other: Ad Board; Beigene: Speakers Bureau; Verastem: Speakers Bureau. Christian: Millenium: Other: Institution: Research Grant/Funding; Triphase: Other: Institution: Research Grant/Funding; Celgene/BMS: Other: Institution: Research Grant/Funding; Acerta: Other: Institution: Research Grant/Funding; Seattle Genetics: Consultancy, Other: Institution: Research Grant/Funding; Genentech: Consultancy, Other: Institution: Research Grant/Funding; AstraZeneca: Consultancy; VeraStem: Consultancy; Morphosys: Consultancy, Other: Institution: Research Grant/Funding; Immunomedics: Other: Institution: Research Grant/Funding; Merck: Other: Institution: Research Grant/Funding. Baiocchi: Prelude Therapeutics: Consultancy; Atara Biotherapeutics: Consultancy; Codiak Biosciences: Research Funding; viracta: Consultancy, Current holder of stock options in a privately-held company. Maddocks: Karyopharm: Divested equity in a private or publicly-traded company in the past 24 months; Morphosys: Divested equity in a private or publicly-traded company in the past 24 months; ADC Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months; Beigene: Divested equity in a private or publicly-traded company in the past 24 months; KITE: Divested equity in a private or publicly-traded company in the past 24 months; Celgene: Divested equity in a private or publicly-traded company in the past 24 months; Pharmacyclics: Divested equity in a private or publicly-traded company in the past 24 months; BMS: Divested equity in a private or publicly-traded company in the past 24 months; Merck: Divested equity in a private or publicly-traded company in the past 24 months; Novatis: Divested equity in a private or publicly-traded company in the past 24 months; Janssen: Divested equity in a private or publicly-traded company in the past 24 months; Seattle Genetics: Divested equity in a private or publicly-traded company in the past 24 months. Sawalha: Epizyme: Consultancy; BeiGene: Research Funding; Celgene/BMS: Research Funding; TG Therapeutics: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal